Protocol for molecular detection of mollicutes in maize
Zea mays

- Productive system:
- system: Maize
- Geographic coverage:
-
Inter-Andean Valleys, Orinoquía, Caribbean and Pacific regions

Description
Maize stunt disease is caused by the mollicutes Candidatus Phytoplasma asteris or MSBP (Maize Bushy Stunt Phytoplasma) and Spiroplasma kunkelii or CSS (Corn Stunt Spiroplasm), which are transmitted to plants by the leafhopper Dalbulus maidis, which is its vector. This disease has become the leading health limitation of this crop, both for grain and silage production, affecting the average yield at harvest. The disease is already found in different maize-producing regions in Colombia, where the need for diagnostic tools that allow early detection of pathogens in adult Dalbulus maidis insects and in maize plant tissue has been evidenced to make decisions on agronomic management of the crop; thus, obtain economically profitable yields for the farmer.
With this protocol, it is possible to molecularly detect the two mollicutes Candidatus Phytoplasma asteris (MSBP) and Spiroplasma kunkelii (CSS), from plant tissue and adult insects of Dalbulus maidis collected in the field.
The recommendation is to carry out two detections of mollicutes for each production cycle: one in Dalbulus maidis insects prior to establishing the crop and another in plant tissue 40 days after plants emerge. A sample will be composed of the collection of plant tissue or vector insects from five points in one hectare so that it is representative.
Having a timely diagnosis of insects or plants allows the farmer to implement management and control strategies for the disease, to be able to reach the harvest stage. In the same way, it allows determining if the pathogens are present within Dalbulus maidis adults and if the latter are risk factors in the area to be cultivated, allowing the implementation of the necessary measures to mitigate their effect in maize-producing areas.
Early disease diagnosis allows producers and control entities to establish a crop management plan. It will also allow monitoring-based applications, which reduce the excessive use of agrochemicals to control the vector.
How do you collect the plant tissue sample for analysis?
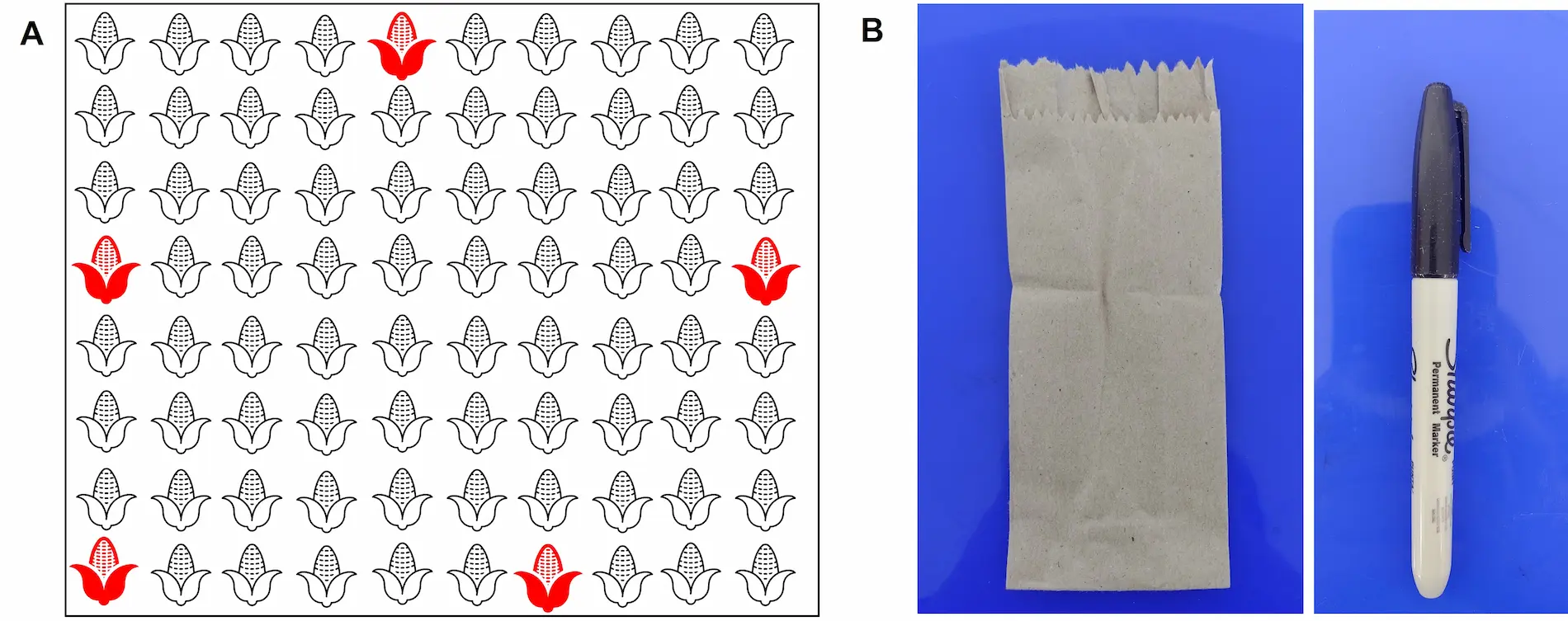
For plant tissue sampling, it is essential to collect samples from plants located at the edge of the field because there is a relationship between the entry into the field, movement, and transmission of mollicutes by the vector insect Dalbulus maidis to the plants (Fig. 1A). Additionally, a permanent marker and paper bags of approximately 15 cm x 6 cm should be available (Fig. 1B).
Sampling time
Sampling should be done at the vegetative stage of the maize crop. That is when the plants have from 3 fully developed leaves (V3) to 10 fully developed leaves (V10), ideally before flowering (Fig. 2B), since this stage of development would correspond to a late development phase of the plant to make the diagnosis. This is because, in case of a positive result, it will not be possible to implement strategies to manage the disease and avoid a high percentage of the crop being affected (Fig. 2).

Field sample collection and packaging to prevent degradation
Five (5) plants must be selected in the field according to Figure 1A. From each plant, locate a leaf from the middle third, from which a tissue fragment of approximately 10 cm x 5 cm in diameter, including the central rib, will be cut. Subsequently, the fragments collected from each plant must be deposited completely and extended inside the same paper bag. The bag must be marked externally with the sample identification (Fig. 3).

How do you collect insect samples for analysis?
To sample adult Dalbulus maidis insects, it is important to carry out sampling before the establishment of the lot in adjacent fields and weeds at the edge of the field (Fig. 5) or in the emergence stages of the crop (Fig. 2A). Carrying out sampling and their respective analyses in later stages with a positive result will not allow the implementation of management strategies to control the insect vector that reduces the dissemination of mollicutes in the plot.
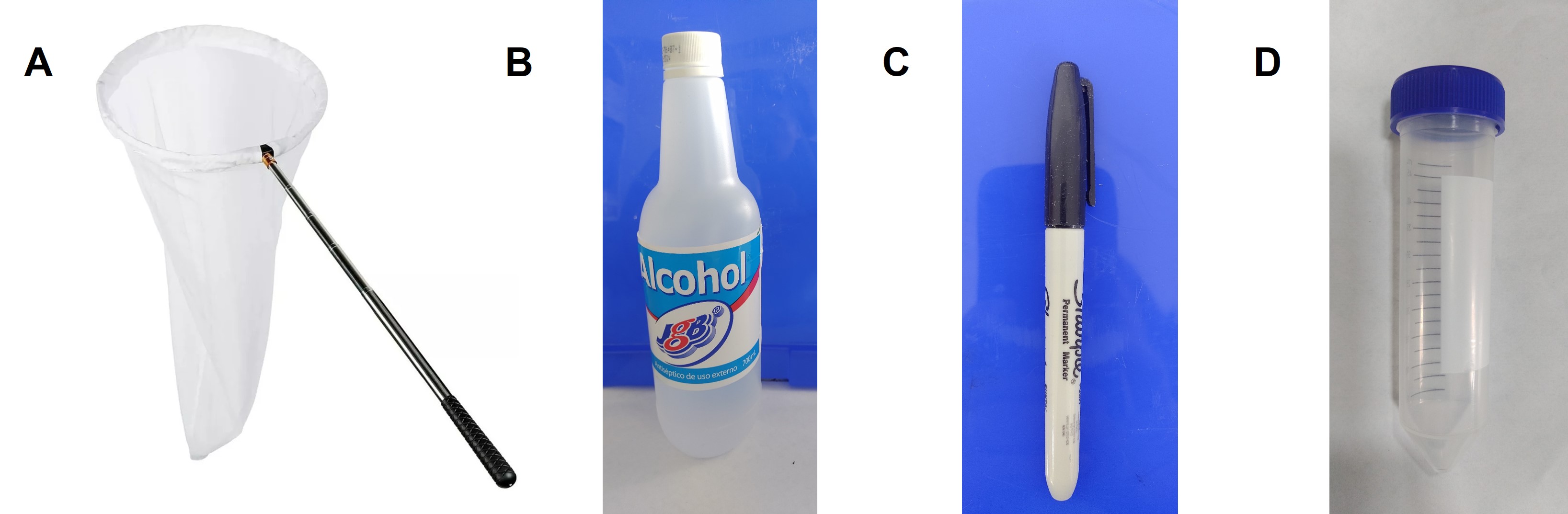
Sampling due to the required materials and sample conditioning for detection is recommended to be carried out by the agronomist or farmer's technical assistant. For sampling, the following are necessary: an entomological needle, a permanent marker, 70% antiseptic alcohol, and tubes or plastic containers with a capacity of 15 to 50 ml. (Fig. 4).
Sampling
The collection is carried out by making 5 double passes of the entomological net (Jama) in one linear meter of the field with five repetitions (Fig. 5). After making the 10 double passes with the entomological net, the sample must be concentrated at its base (Fig. 6A). Introduce the base of the entomological net into the vial with alcohol and then collect by scraping in a tube or plastic container with a capacity of 15 to 20 ml with 70% antiseptic alcohol (Fig. 6B and 6C). The samples collected in the field, until they are conditioned, can be stored under refrigeration conditions (4–10°C).

Packaging samples for transportation
Once the samples have been collected in the field, it is necessary to select the adults morphologically related to the Dalbulus genus and prepare them for transportation. For this selection and cleaning process, 2 ml Eppendorf tubes, 70% antiseptic alcohol, a paintbrush number zero, an HB No. 2 pencil, scissors, a straight handle, entomological tweezers, a Pasteur pipette and opaline paper are required (Fig. 7).
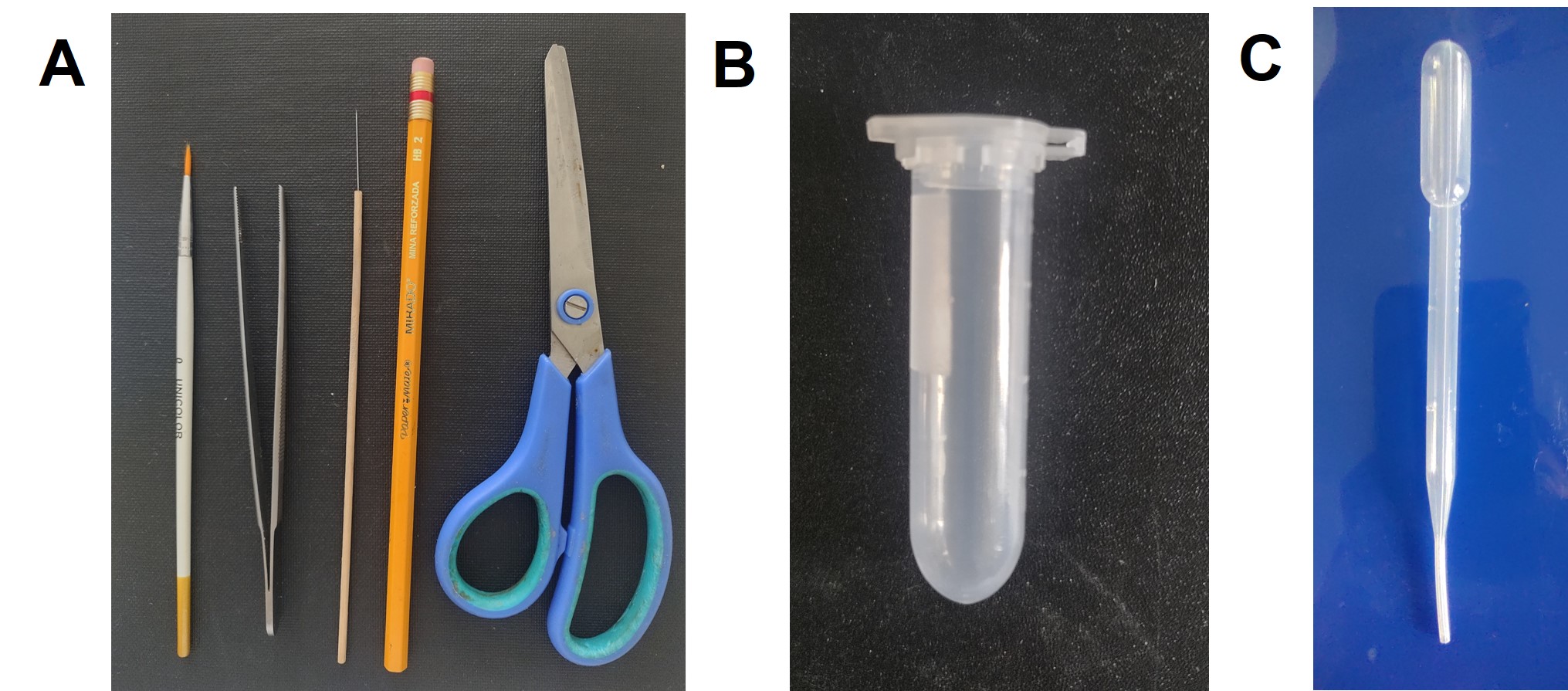
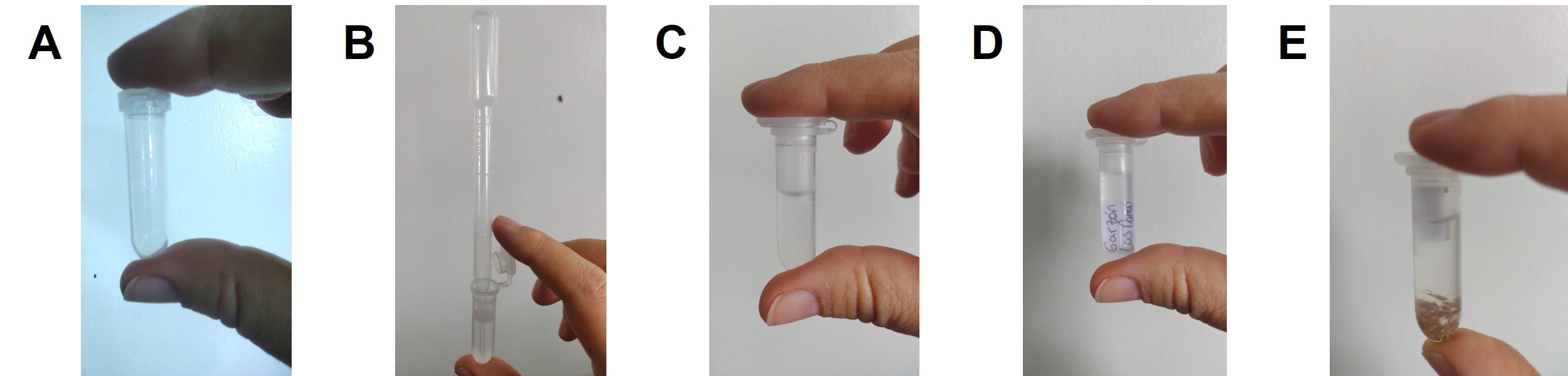
Add 1 ml of 70% antiseptic alcohol to a 2 ml Eppendorf tube with a Pasteur pipette. Then, insert an opaline paper label marked with a pencil where the sample is identified. Finally, dispense the sample collected in the 15 to 50 ml plastic tube or container from the field into a Petri dish, using the straight handle and the brush to select the adults morphologically associated with the genus Dalbulus, and introduce them into the tube (Fig. 8).
Publications
- Article: Design of a polymerase chain reaction for specific detection of corn stunt spiroplasma. Plant Disease, 85(5), 475–480.
- Article: PCR assay for detection of the phytoplasma associated with maize bushy stunt disease. Plant Disease, 80(3), 263–269
Image gallery









